
Chemistry, 05.05.2020 16:44 ladnerhailey16
An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M O2. At this temperature, Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g). What is the equilibrium concentration of CO? An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M O2. At this temperature, Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g). What is the equilibrium concentration of CO? 3.1 × 10-1 M 4.8 × 10-6 M 2.2 × 10-3 M 1.9 × 10 7 M 9.3 × 10-2 M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1


Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M...
Questions

Biology, 10.06.2021 22:40


Mathematics, 10.06.2021 22:40

Social Studies, 10.06.2021 22:40


Geography, 10.06.2021 22:40



Mathematics, 10.06.2021 22:40


Mathematics, 10.06.2021 22:40


Biology, 10.06.2021 22:40

Biology, 10.06.2021 22:40


Mathematics, 10.06.2021 22:40

Mathematics, 10.06.2021 22:40



Mathematics, 10.06.2021 22:40

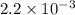 M.
M.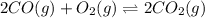
![K_{c}=\frac{[CO_{2}]^{2}}{[CO]^{2}[O_{2}]}](/tpl/images/0640/3657/11981.png)
![[CO_{2}]](/tpl/images/0640/3657/0a7e9.png) ,
, ![[CO]](/tpl/images/0640/3657/32558.png) and
and ![[O_{2}]](/tpl/images/0640/3657/9a638.png) represent equilibrium concentration of
represent equilibrium concentration of 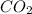 , CO and
, CO and 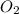 respectively.
respectively.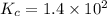
![[CO]=\sqrt{\frac{[CO_{2}]^{2}}{K_{c}.[O_{2}]}}](/tpl/images/0640/3657/02c20.png) M =
M = 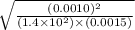 M =
M = 

