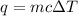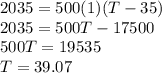If 2,035 cal of heat is added to a 500.0 g sample of water at 35.0°C, what is the final
temper...

Chemistry, 05.05.2020 10:45 maddie0533
If 2,035 cal of heat is added to a 500.0 g sample of water at 35.0°C, what is the final
temperature of the water?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
Questions


Engineering, 17.09.2021 09:40

Mathematics, 17.09.2021 09:40



Computers and Technology, 17.09.2021 09:40

Mathematics, 17.09.2021 09:40


English, 17.09.2021 09:40


Mathematics, 17.09.2021 09:40



Computers and Technology, 17.09.2021 09:40

Chemistry, 17.09.2021 09:40

Mathematics, 17.09.2021 09:40


English, 17.09.2021 09:40