
Chemistry, 05.05.2020 09:46 kezionhoward13
Identify the correct set-up. How many moles of aluminum oxide would form if 25.0 g of aluminum react completely given the following chemical reaction: 4Al + 3O₂ → 2Al₂O₃?
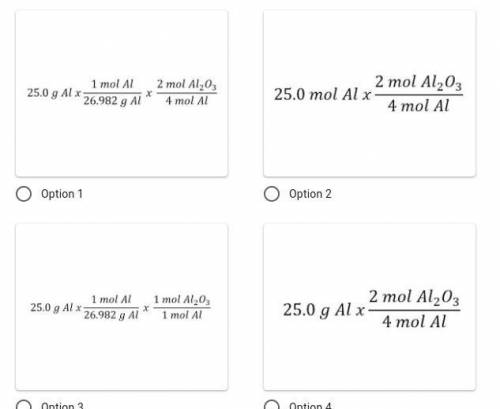

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1


Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
Identify the correct set-up. How many moles of aluminum oxide would form if 25.0 g of aluminum react...
Questions

History, 22.09.2019 09:30





Mathematics, 22.09.2019 09:30

English, 22.09.2019 09:30

Mathematics, 22.09.2019 09:30


Mathematics, 22.09.2019 09:30


English, 22.09.2019 09:30



Mathematics, 22.09.2019 09:30


History, 22.09.2019 09:30

Social Studies, 22.09.2019 09:30

Chemistry, 22.09.2019 09:30

Mathematics, 22.09.2019 09:30



