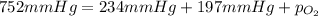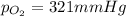A mixture of helium, nitrogen, and oxygen has a total pressure of 752 mm Hg. The
partial press...

Chemistry, 05.05.2020 05:42 tylorroundy
A mixture of helium, nitrogen, and oxygen has a total pressure of 752 mm Hg. The
partial pressures of helium and nitrogen are 234 mm Hg and 197 mm Hg, respectively.
What is the partial pressure of oxygen in the mixture?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1


You know the right answer?
Questions


Business, 13.02.2021 14:00



Mathematics, 13.02.2021 14:00


Mathematics, 13.02.2021 14:00

Mathematics, 13.02.2021 14:00



Mathematics, 13.02.2021 14:00

Arts, 13.02.2021 14:00

Mathematics, 13.02.2021 14:00

French, 13.02.2021 14:00

World Languages, 13.02.2021 14:00

Social Studies, 13.02.2021 14:00

History, 13.02.2021 14:00

Chemistry, 13.02.2021 14:00


Mathematics, 13.02.2021 14:00

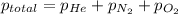
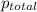 = total pressure of gases = 752 mm Hg
= total pressure of gases = 752 mm Hg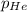 = partial pressure of Helium = 234 mm Hg
= partial pressure of Helium = 234 mm Hg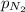 = partial pressure of nitrogen = 197 mm Hg
= partial pressure of nitrogen = 197 mm Hg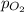 = partial pressure of oxygen = ?
= partial pressure of oxygen = ?