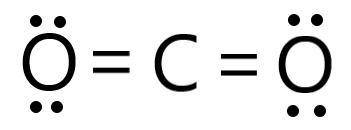Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Determine the electron geometry (eg) and molecular geometry (mg) of CO2.
eg=trigonal planar, mg...
eg=trigonal planar, mg...
Questions


History, 20.04.2021 22:30


Mathematics, 20.04.2021 22:30

Mathematics, 20.04.2021 22:30

English, 20.04.2021 22:30


Chemistry, 20.04.2021 22:30



Mathematics, 20.04.2021 22:30



Mathematics, 20.04.2021 22:30


Mathematics, 20.04.2021 22:30



English, 20.04.2021 22:30