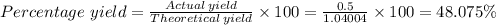100.0mL of 0.40M Al(NO3)3 is reacted with 100.0 mL of 0.40M Al(OH)3 according to
the following reaction: Al(NO3)3 + 3KOH → Al(OH)3 + 3KNO3
1) Identify the limiting reactant.
2) Identify the excess reactant.
3) Determine the theoretical yield of Al(OH)3
4) How much excess reactant is left over after the reaction is complete?
5) If 0.50g of Al(OH)3 determine the percent yield of Al(OH)3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
100.0mL of 0.40M Al(NO3)3 is reacted with 100.0 mL of 0.40M Al(OH)3 according to
the following...
the following...
Questions

English, 14.01.2021 04:00


Mathematics, 14.01.2021 04:00





Geography, 14.01.2021 04:00

English, 14.01.2021 04:00

Mathematics, 14.01.2021 04:00



Mathematics, 14.01.2021 04:00

Advanced Placement (AP), 14.01.2021 04:00



Mathematics, 14.01.2021 04:00


Biology, 14.01.2021 04:00