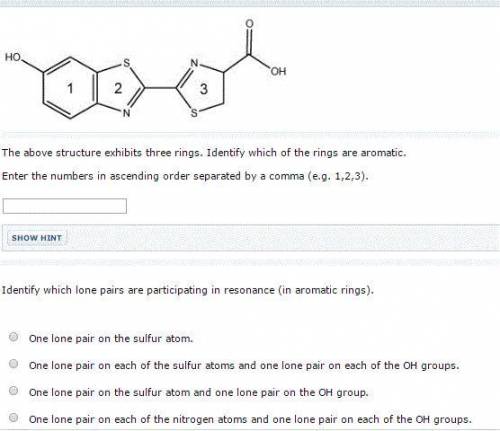
Chemistry, 05.05.2020 00:29 stefaniethibodeaux
The above structure exhibits three rings. Identify which of the rings are aromatic. Enter the numbers in ascending order separated by a comma (e. g. 1,2,3). Identify which lone pairs are participating in resonance (in aromatic rings). One lone pair on each of the nitrogen atoms and one lone pair on each of the OH groups. One lone pair on the sulfur atom. One lone pair on the sulfur atom and one lone pair on the OH group. One lone pair on each of the sulfur atoms and one lone pair on each of the OH groups

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
The above structure exhibits three rings. Identify which of the rings are aromatic. Enter the number...
Questions

Mathematics, 05.05.2020 11:06

Mathematics, 05.05.2020 11:06


Mathematics, 05.05.2020 11:06



Mathematics, 05.05.2020 11:06

Chemistry, 05.05.2020 11:06


Mathematics, 05.05.2020 11:06









Mathematics, 05.05.2020 11:06