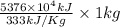On a hot summer day you and some friends decide you want to cool down your pool. Determine the mass of ice you would need to add to bring the equilibrium temperature of the system to 300K. The pool contains 400,000 L (at a density of 1 kg/L) of water initially at 305K. Assume the ice is at 0°C (273K), the heat capacity of water is 4.2 J/(g*K), and the enthalpy of melting ice is 333 kJ/kg.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
On a hot summer day you and some friends decide you want to cool down your pool. Determine the mass...
Questions

Mathematics, 20.04.2021 16:10






Advanced Placement (AP), 20.04.2021 16:10

History, 20.04.2021 16:10


Mathematics, 20.04.2021 16:10



Mathematics, 20.04.2021 16:10

Mathematics, 20.04.2021 16:10



Mathematics, 20.04.2021 16:10


Mathematics, 20.04.2021 16:10

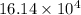 kg.
kg.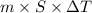
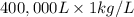
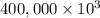 g (as 1 kg = 1000 g)
g (as 1 kg = 1000 g) = 305 K - 273 K
= 305 K - 273 K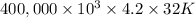
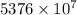 J
J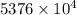 kJ
kJ