Chemistry, 05.05.2020 14:49 hosteenimport21
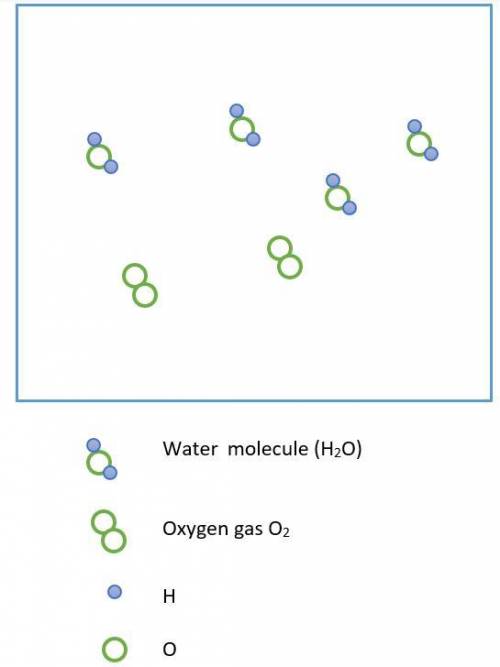
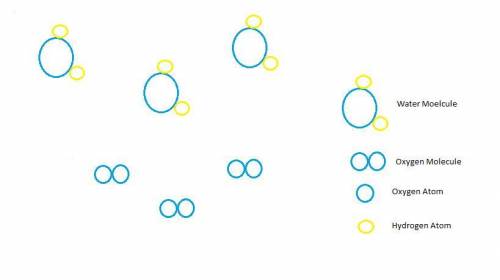
Hydrogen peroxide, H2O2, decomposes according to the equation above. This reaction is thermodynamically favorable at room temperature.
(a) A particulate representation of the reactants is shown below in the box on the left. In the box below on the right, draw the particulate representation of all the molecules that would be produced from these four reactant molecules.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Hydrogen peroxide, H2O2, decomposes according to the equation above. This reaction is thermodynamica...
Questions


Biology, 16.12.2020 21:00



Mathematics, 16.12.2020 21:00




Mathematics, 16.12.2020 21:00



Mathematics, 16.12.2020 21:00

Advanced Placement (AP), 16.12.2020 21:00



Social Studies, 16.12.2020 21:00

Mathematics, 16.12.2020 21:00

Social Studies, 16.12.2020 21:00

Mathematics, 16.12.2020 21:00