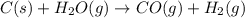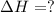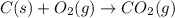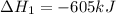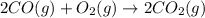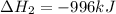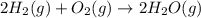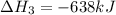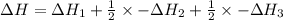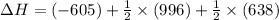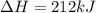Chemistry, 05.05.2020 14:47 clarajeansonels9987
QUESTION 13
Use the standard reaction enthalpies given below to determine AHºrn for the followi
C(s) + H2O(g) - CO(g) + H2(9)
Given:
Reaction 1: C(s) + O2(g) – CO2(g) AHºrxn = -605 kJ
Reaction 2: 2 CO(g) + O2(g) – 2 CO2(g) AH°x = -966 kJ
Reaction 3: 2 H2(g) + O2(g) → 2 H2O(g) AHⓇx = -638 kJ

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
QUESTION 13
Use the standard reaction enthalpies given below to determine AHºrn for the followi...
Use the standard reaction enthalpies given below to determine AHºrn for the followi...
Questions

Social Studies, 18.12.2020 03:10

History, 18.12.2020 03:10

Mathematics, 18.12.2020 03:10

Mathematics, 18.12.2020 03:10

History, 18.12.2020 03:10

Mathematics, 18.12.2020 03:10



Mathematics, 18.12.2020 03:10


Mathematics, 18.12.2020 03:10


History, 18.12.2020 03:10

Mathematics, 18.12.2020 03:10

Mathematics, 18.12.2020 03:10


Mathematics, 18.12.2020 03:10

Mathematics, 18.12.2020 03:10

English, 18.12.2020 03:10

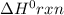 for the reaction is 212 kJ
for the reaction is 212 kJ