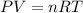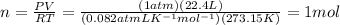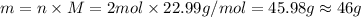Chemistry, 09.10.2019 09:30 nellyjsotelo
According to the equation 2na + 2h2o mc012-1.jpg 2naoh+h2, what mass of na is required to yield 22.4 l of h2 at stp? (the atomic mass of na is 22.99 a. 1.00 g. b. 2.00 g. c. 23.0 g. d. 46.0 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3


Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
According to the equation 2na + 2h2o mc012-1.jpg 2naoh+h2, what mass of na is required to yield 22.4...
Questions

Mathematics, 18.07.2020 21:01





Mathematics, 18.07.2020 21:01


Mathematics, 18.07.2020 21:01

Mathematics, 18.07.2020 21:01



Mathematics, 18.07.2020 21:01








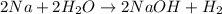
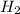 formed at STP,
formed at STP,