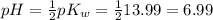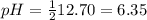Chemistry, 05.05.2020 00:56 kell22wolf
9-19. Calculate the pH of water at 25°C and 75°C. The values for pKw at these temperatures are 13.99 and 12.70, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
9-19. Calculate the pH of water at 25°C and 75°C. The values for pKw at these temperatures are 13.99...
Questions

Mathematics, 21.03.2021 09:10






Mathematics, 21.03.2021 09:20


Mathematics, 21.03.2021 09:20

Business, 21.03.2021 09:20

English, 21.03.2021 09:20

History, 21.03.2021 09:20

Mathematics, 21.03.2021 09:20


Mathematics, 21.03.2021 09:20

English, 21.03.2021 09:20

Mathematics, 21.03.2021 09:20

Mathematics, 21.03.2021 09:20

Mathematics, 21.03.2021 09:20

![K_{w} = [H_{3}O^{+}]*[OH^{-}]](/tpl/images/0630/9121/6c957.png)
![K_{w} = [H_{3}O^{+}]^{2}](/tpl/images/0630/9121/1f3e0.png)
![[H_{3}O^{+}] = \sqrt{K_{w}}](/tpl/images/0630/9121/17fd1.png) (1)
(1)![pH = -log([H_{3}O^{+}])](/tpl/images/0630/9121/6ab72.png) (2)
(2)![pH = -log([H_{3}O^{+}]) = -log(\sqrt{K_{w}})](/tpl/images/0630/9121/66671.png) (3)
(3)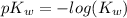
 (4)
(4)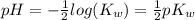 (5)
(5)