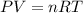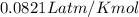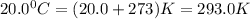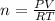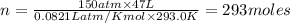Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
How many moles of Oxygen (O2) gas can be stored in a standard gas cylinder at a temperature of 20.0o...
Questions





Mathematics, 23.04.2020 20:38




Mathematics, 23.04.2020 20:38


Mathematics, 23.04.2020 20:38

English, 23.04.2020 20:38

History, 23.04.2020 20:38

Social Studies, 23.04.2020 20:38

English, 23.04.2020 20:38

Mathematics, 23.04.2020 20:38




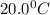 and a pressure of 15200 kPa
and a pressure of 15200 kPa