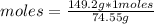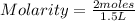Chemistry, 05.05.2020 01:29 Natalierg05
What is the molarity of a solution of KCl if 1500. mL contains 149.2 grams of KCl? (Atomic mass of K = 39.10 g/mol and Cl = 35.45 g/mol)

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
What is the molarity of a solution of KCl if 1500. mL contains 149.2 grams of KCl? (Atomic mass of K...
Questions

History, 22.07.2021 01:30



History, 22.07.2021 01:30

Mathematics, 22.07.2021 01:30


Mathematics, 22.07.2021 01:30





Mathematics, 22.07.2021 01:30

Social Studies, 22.07.2021 01:30







Mathematics, 22.07.2021 01:30

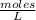
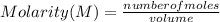
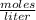 ).
).