
Chemistry, 05.05.2020 01:18 victoriavacodos
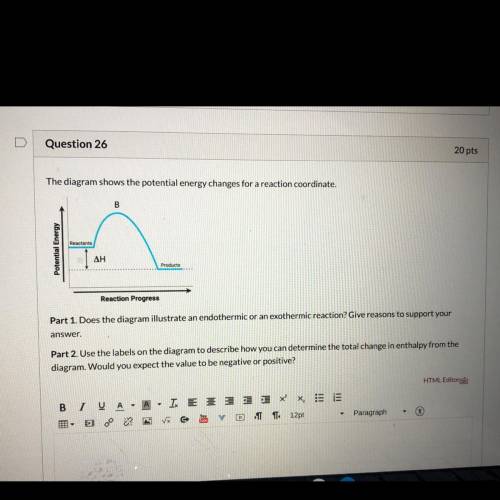
Part 1 : does the diagram illustrate an endothermic or exothermic reaction? Give reasons to support your answer.
Part 2: Use the labels on the diagram to describe how you can determine he total change in enthalpy from the diagram. Would you expect the value to be negative or positive?

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3
You know the right answer?
Part 1 : does the diagram illustrate an endothermic or exothermic reaction? Give reasons to support...
Questions

History, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50


Mathematics, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50

Spanish, 22.01.2021 01:50



English, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50

Physics, 22.01.2021 01:50

Arts, 22.01.2021 01:50

English, 22.01.2021 01:50



Computers and Technology, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50



