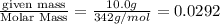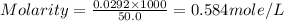Chemistry, 05.05.2020 01:06 Fireburntbudder
What is the molarity of a 50.0 ml solution containing 10.0 grams of table sugar (C12H22O11)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1


Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

You know the right answer?
What is the molarity of a 50.0 ml solution containing 10.0 grams of table sugar (C12H22O11)?...
Questions






Mathematics, 17.07.2019 08:30










Arts, 17.07.2019 08:30


Social Studies, 17.07.2019 08:30


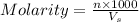
 = volume of solution in ml
= volume of solution in ml