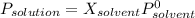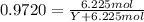Chemistry, 05.05.2020 04:23 NylaJohn29
The common laboratory solvent ethanol is often used to purify substances dissolved in it. The vapor pressure of ethanol , CH3CH2OH, is 54.68 mm Hg at 25 °C. In a laboratory experiment, students synthesized a new compound and found that when 32.83 grams of the compound were dissolved in 286.8 grams of ethanol, the vapor pressure of the solution was 53.15 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight of this compound? ethanol = CH3CH2OH = 46.07 g/mol.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
The common laboratory solvent ethanol is often used to purify substances dissolved in it. The vapor...
Questions




Mathematics, 28.01.2020 11:31


History, 28.01.2020 11:31

Biology, 28.01.2020 11:31

English, 28.01.2020 11:31


History, 28.01.2020 11:31

English, 28.01.2020 11:31


Mathematics, 28.01.2020 11:31

English, 28.01.2020 11:31





Mathematics, 28.01.2020 11:31