
Consider the two reduction half-reactions: Na+(aq) + e− LaTeX: \longrightarrow⟶ Na(s) Eo = −2.71 V Cl2(g) + 2 e− LaTeX: \longrightarrow⟶ 2 Cl−(aq) Eo = 1.36 VUse the electrode potentials above to calculate Eocell and ∆Gorxn for the reaction below, and determine if it is the reaction for a voltaic cell or an electrolytic cell. 2 Na+(aq) + 2 Cl−(aq) LaTeX: \longrightarrow⟶2 Na(s) + Cl2(g)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2


Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Consider the two reduction half-reactions: Na+(aq) + e− LaTeX: \longrightarrow⟶ Na(s) Eo = −2.71 V C...
Questions


Biology, 09.01.2021 02:40

Health, 09.01.2021 02:40

Mathematics, 09.01.2021 02:40


History, 09.01.2021 02:40


Mathematics, 09.01.2021 02:40



English, 09.01.2021 02:40


Mathematics, 09.01.2021 02:40

Mathematics, 09.01.2021 02:40



Chemistry, 09.01.2021 02:40

Mathematics, 09.01.2021 02:40


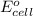 = -4.07v
= -4.07v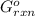 = +785.510 KJ
= +785.510 KJ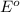 and Δ
and Δ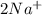 +
+ 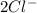 →
→ 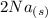 +
+ 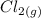
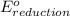 - Δ
- Δ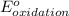 = -2.71v - 1.36v = -4.07V
= -2.71v - 1.36v = -4.07V

