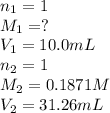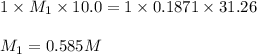G Suppose you are titrating vinegar, which is an acetic acid solution of unknown strength, with a sodium hydroxide solution according to the equation H C 2 H 3 O 2 + N a O H ⟶ H 2 O + N a C 2 H 3 O 2 If you require 31.26 mL of 0.1871 M N a O H solution to titrate 10.0 mL of H C 2 H 3 O 2 solution, what is the concentration of acetic acid in the vinegar?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
G Suppose you are titrating vinegar, which is an acetic acid solution of unknown strength, with a so...
Questions


Geography, 02.12.2020 21:50

History, 02.12.2020 21:50


Mathematics, 02.12.2020 21:50

Mathematics, 02.12.2020 21:50


English, 02.12.2020 21:50


Mathematics, 02.12.2020 21:50

Mathematics, 02.12.2020 21:50

Mathematics, 02.12.2020 21:50



Mathematics, 02.12.2020 21:50

Chemistry, 02.12.2020 21:50


Mathematics, 02.12.2020 21:50


Mathematics, 02.12.2020 21:50

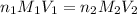
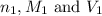 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 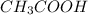
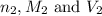 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.