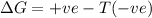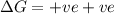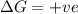Chemistry, 05.05.2020 06:34 Ryleetarver
Use the reaction data in the table below to select the answer choice that best describes this reaction.
Reaction Enthalpy Change
345.7 kJ/mol
Reaction Entropy Change
-25. 3 J/molK
This reaction is never spontaneous.
This reaction is spontaneous at all temperatures.
This reaction is spontaneous at low temperatures.
This reaction is spontaneous at high temperatures

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2


Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
Use the reaction data in the table below to select the answer choice that best describes this reacti...
Questions

Mathematics, 23.09.2021 14:10

Biology, 23.09.2021 14:10


English, 23.09.2021 14:10

German, 23.09.2021 14:10


Chemistry, 23.09.2021 14:10


Chemistry, 23.09.2021 14:10



Mathematics, 23.09.2021 14:10


Chemistry, 23.09.2021 14:10

Mathematics, 23.09.2021 14:10

History, 23.09.2021 14:10

Chemistry, 23.09.2021 14:10


Mathematics, 23.09.2021 14:10

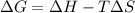
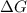 = Gibb's free energy change
= Gibb's free energy change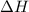 = enthalpy change
= enthalpy change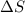 = entropy change
= entropy change