
Chemistry, 05.05.2020 06:28 ricksterv5000
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2(s) + 2H2O(l)C2H2(g) + Ca(OH)2(aq) The product gas, C2H2, is collected over water at a temperature of 25 °C and a pressure of 749 mm Hg. If the wet C2H2 gas formed occupies a volume of 5.87 L, the number of moles of CaC2 reacted was mol. The vapor pressure of water is 23.8 mm Hg at 25 °C. Submit Answer

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2...
Questions



Mathematics, 04.12.2020 17:20

Mathematics, 04.12.2020 17:20




History, 04.12.2020 17:20

Mathematics, 04.12.2020 17:20



Mathematics, 04.12.2020 17:20

English, 04.12.2020 17:20

English, 04.12.2020 17:20




Mathematics, 04.12.2020 17:20

Mathematics, 04.12.2020 17:20

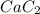 reacted was 0.229
reacted was 0.229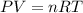
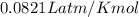
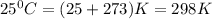
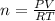
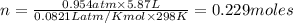
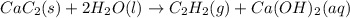
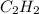 is produced by = 1 mole of
is produced by = 1 mole of 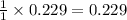 moles of
moles of 

