
Chemistry, 05.05.2020 06:06 wubzwaters
Consider this system at equilibrium. A(aq)↽−−⇀B(aq)ΔH=+750 kJ/mol A(aq)↽−−⇀B(aq)ΔH=+750 kJ/mol What can be said about Q and K immediately after an increase in temperature? Q < K because Q decreased. Q = K because neither changed. Q < K because K increased. Q > K because K decreased. Q > K because Q increased. How will the system respond to a temperature increase? shift left shift right no change

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
Consider this system at equilibrium. A(aq)↽−−⇀B(aq)ΔH=+750 kJ/mol A(aq)↽−−⇀B(aq)ΔH=+750 kJ/mol What...
Questions



Mathematics, 31.08.2020 01:01






Mathematics, 31.08.2020 01:01



Geography, 31.08.2020 01:01








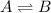
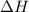 is positive. This signifies that the given reaction is endothermic. So, with increase in temperature, reaction equilibrium shift towards right in accordance with Le-chatelier principle.
is positive. This signifies that the given reaction is endothermic. So, with increase in temperature, reaction equilibrium shift towards right in accordance with Le-chatelier principle.![K_{c}=\frac{[B]}{[A]}](/tpl/images/0633/9882/2d52b.png) where [B} and [A} are equilibrium concentrations of B and A respectively.
where [B} and [A} are equilibrium concentrations of B and A respectively.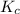 will increase with increase in temperature.
will increase with increase in temperature.

