Chemistry, 05.05.2020 06:08 ismailhajisaid29101
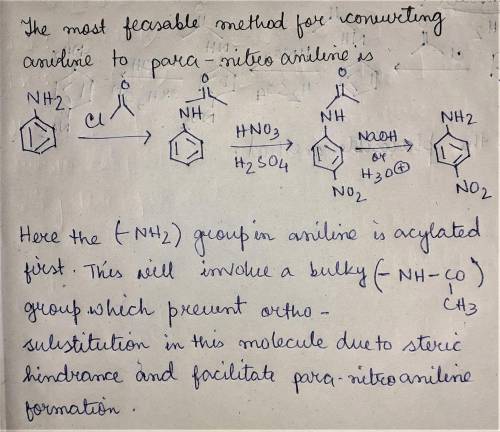
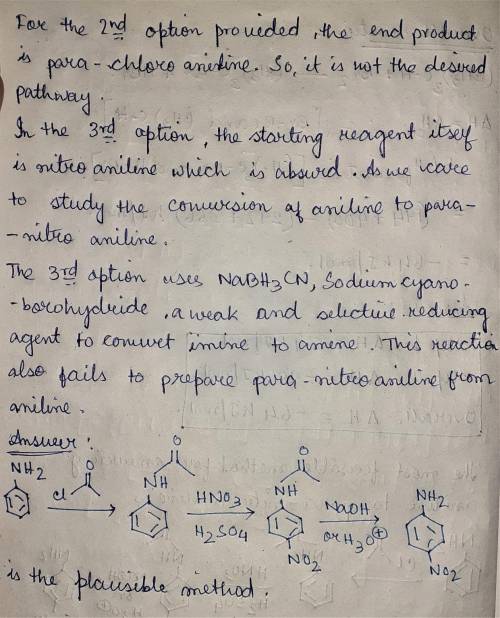
When aniline is treated with a mixture of nitric acid and sulfuric acid, the expected nitration product (para-nitroaniline) is obtained in poor yield. Instead, the major product from nitration is meta-nitroaniline. Apparently, the amino group is protonated under these acidic conditions, and the resulting ammonium group is a meta-director, rather than an ortho-para director. Propose a plausible method for converting aniline into para-nitroaniline.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
When aniline is treated with a mixture of nitric acid and sulfuric acid, the expected nitration prod...
Questions






Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01