Chemistry, 05.05.2020 05:58 CoolRahim9090
G Copper (II) Sulfate forms several hydrates with the general formula CuSO4 times xH2O, where x is an integer. If the hydrate is heated, the water can be drive off leaving pure CuSO4 behind. Suppose a sample of a certain hydrate is heated until all water is removed, and its found that the mass of the sample decreases by 31%. Which hydrate is it? THat is, WHAT IS X?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3


Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
You know the right answer?
G Copper (II) Sulfate forms several hydrates with the general formula CuSO4 times xH2O, where x is a...
Questions

English, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00


Biology, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00


History, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00


History, 20.11.2020 01:00


Mathematics, 20.11.2020 01:00


Mathematics, 20.11.2020 01:00


Mathematics, 20.11.2020 01:00

Biology, 20.11.2020 01:00


Mathematics, 20.11.2020 01:00

Chemistry, 20.11.2020 01:00

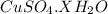
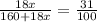
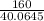 = 3.99 ≅ 4.
= 3.99 ≅ 4.