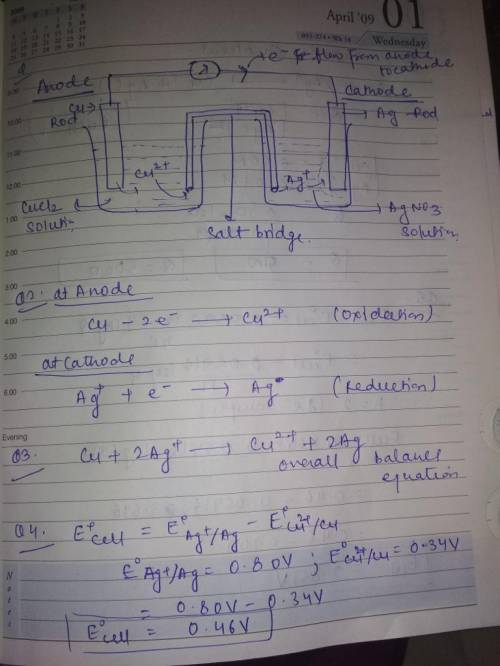
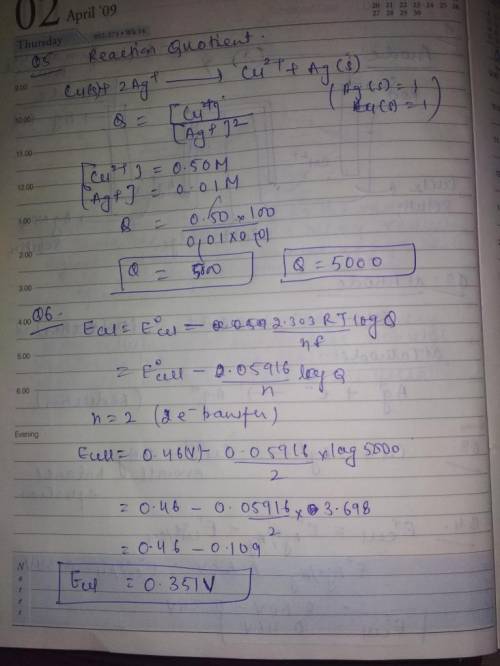Cu(s), CuCl2 (0.50 M) || Ag(s), AgNO3 (0.010 M) 1.
Draw the schematic of the electroche...

Chemistry, 05.05.2020 09:05 jamarian101
Cu(s), CuCl2 (0.50 M) || Ag(s), AgNO3 (0.010 M) 1.
Draw the schematic of the electrochemical cell that you created including all the components (metals, solutions, salt bridges, voltmeters, etc.) in this portion of the experiment. Annotate on the schematic which side is the anode, which side is the cathode, the sign of each half cell, the composition of the metals and solutions, and the direction of the flow of the electrons through the cell.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Questions


Mathematics, 26.02.2021 23:10

Chemistry, 26.02.2021 23:10

Mathematics, 26.02.2021 23:10

English, 26.02.2021 23:10



Mathematics, 26.02.2021 23:10

Mathematics, 26.02.2021 23:10

Mathematics, 26.02.2021 23:10

Business, 26.02.2021 23:10


Mathematics, 26.02.2021 23:10

Mathematics, 26.02.2021 23:10


Arts, 26.02.2021 23:10


Mathematics, 26.02.2021 23:10

Mathematics, 26.02.2021 23:10

Mathematics, 26.02.2021 23:10