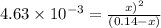Chemistry, 05.05.2020 08:58 Kategaldamez3
The equilibrium constant for the reaction
COCl2 (g) CO (g) + Cl2 (g) is Kc = 4.63 ´ 10–3 at 527 °C
If 10 g of COCl2(g) is placed in a 1 L container, determine how much Cl2 is present at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
The equilibrium constant for the reaction
COCl2 (g) CO (g) + Cl2 (g) is Kc = 4.63 ´ 10–3 at 52...
COCl2 (g) CO (g) + Cl2 (g) is Kc = 4.63 ´ 10–3 at 52...
Questions

English, 11.10.2019 00:20

English, 11.10.2019 00:20

Mathematics, 11.10.2019 00:20

Mathematics, 11.10.2019 00:20




Mathematics, 11.10.2019 00:20


Mathematics, 11.10.2019 00:20




Chemistry, 11.10.2019 00:30

Geography, 11.10.2019 00:30


Geography, 11.10.2019 00:30

Physics, 11.10.2019 00:30

Mathematics, 11.10.2019 00:30

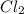 at equilibrium is 0.023 M
at equilibrium is 0.023 M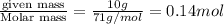
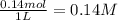
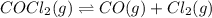
![K_c=\frac{[CO]\times [Cl_2]}{[COCl_2]}](/tpl/images/0635/7088/d6aec.png)