From this combustion equation, 2CH22 + 3102 - 22H,0 + 2000, calculate the liters of
carbon dio...

Chemistry, 05.05.2020 10:11 ashleytellez
From this combustion equation, 2CH22 + 3102 - 22H,0 + 2000, calculate the liters of
carbon dioxide produced when 16.9 grams of CH are combusted

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
4moles of nitrogen gas are confined to a 6.0 l vessel at 177 °c and 12.0 atm. if the vessel is allowed to expand isothermally to 36.0 l, what would be the final pressure?
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Questions

Mathematics, 17.03.2021 23:40

Geography, 17.03.2021 23:40

Biology, 17.03.2021 23:40

History, 17.03.2021 23:40











Mathematics, 17.03.2021 23:40

History, 17.03.2021 23:40

Chemistry, 17.03.2021 23:40

Biology, 17.03.2021 23:40


Geography, 17.03.2021 23:40

 produced are, 26.7 liters.
produced are, 26.7 liters.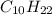

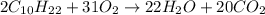
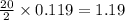 moles of
moles of 

