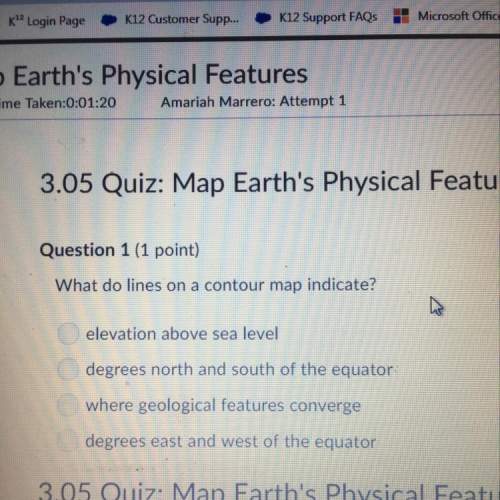
Chemistry, 05.05.2020 11:14 mrfishyyyy
Use the standard enthalpies of formation for the reactants and products to solve for the ΔHrxn for the following reaction. (The ΔHf of HCN is 108.9 kJ/mol, AgCl is -127.068 kJ/mol, HCl is -167.159 kJ/mol and AgCN is 146.0 kJ/mol.)
HCl (aq) + AgCN (s) HCN (aq) + AgCl (s)
ΔHrxn =
The reaction is

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
Use the standard enthalpies of formation for the reactants and products to solve for the ΔHrxn for t...
Questions





Mathematics, 09.02.2021 20:50



Mathematics, 09.02.2021 20:50


Arts, 09.02.2021 20:50

Mathematics, 09.02.2021 20:50

Mathematics, 09.02.2021 20:50

Mathematics, 09.02.2021 20:50


Mathematics, 09.02.2021 20:50