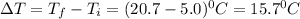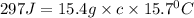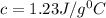Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

You know the right answer?
Calculate the specific heat capacity of a new alloy if a 15.4 g sample absorbs 297 Joules when it is...
Questions

Social Studies, 07.07.2019 23:30

Mathematics, 07.07.2019 23:30

History, 07.07.2019 23:30




Mathematics, 07.07.2019 23:30






Computers and Technology, 07.07.2019 23:30

Biology, 07.07.2019 23:30

English, 07.07.2019 23:30



Mathematics, 07.07.2019 23:30

English, 07.07.2019 23:30

History, 07.07.2019 23:30

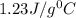
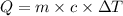
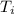 = 5.0°C
= 5.0°C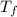 =20.7°C
=20.7°C