
Chemistry, 05.05.2020 13:27 Gghbhgy8716
You burn a 15g jellybean to warm 50 mL of water, which increases the temperature of the water 25 °C. How many calories of heat are transferred from the jellybean to the water? Assume there is no heat loss.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
You burn a 15g jellybean to warm 50 mL of water, which increases the temperature of the water 25 °C....
Questions



History, 18.07.2019 19:00

Mathematics, 18.07.2019 19:00

Mathematics, 18.07.2019 19:00

Mathematics, 18.07.2019 19:00

Mathematics, 18.07.2019 19:00

History, 18.07.2019 19:00


English, 18.07.2019 19:00



Mathematics, 18.07.2019 19:00

Mathematics, 18.07.2019 19:00

Computers and Technology, 18.07.2019 19:00


History, 18.07.2019 19:00



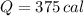
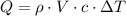
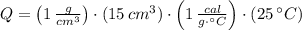
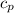 of water= 4.18J/g/°C
of water= 4.18J/g/°C

