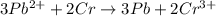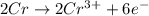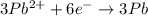Chemistry, 05.05.2020 13:14 madiiiiiii69
Given the balanced ionic equation:
3Pb2+(aq) + 2Cr(s) ---> 3Pb(s) + 2Cr3+(aq)
what is the number of moles of electrons gained by 3.0 moles of lead ions?
1. 5.0 mol
2. 2.0 mol
3. 3.0 mol
4. 6.0 mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Given the balanced ionic equation:
3Pb2+(aq) + 2Cr(s) ---> 3Pb(s) + 2Cr3+(aq)
wh...
3Pb2+(aq) + 2Cr(s) ---> 3Pb(s) + 2Cr3+(aq)
wh...
Questions


Mathematics, 02.03.2021 18:10

Biology, 02.03.2021 18:10



World Languages, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10

History, 02.03.2021 18:10



English, 02.03.2021 18:10


Mathematics, 02.03.2021 18:10

History, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10


Mathematics, 02.03.2021 18:10


Mathematics, 02.03.2021 18:10

Biology, 02.03.2021 18:10