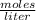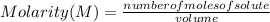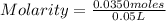What is the molarity of a 50.0 mL solution that contains 0.0350 mol of sodium sulfate, Na2SO4?
...

Chemistry, 05.05.2020 15:22 linreaburg
What is the molarity of a 50.0 mL solution that contains 0.0350 mol of sodium sulfate, Na2SO4?
0.007 mol/L
0.07 mol/L
0.0007 mol/L
0.7 mol/l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3


Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
Questions


Advanced Placement (AP), 06.12.2019 06:31



Mathematics, 06.12.2019 06:31

Health, 06.12.2019 06:31


Mathematics, 06.12.2019 06:31





Mathematics, 06.12.2019 06:31

Mathematics, 06.12.2019 06:31


Mathematics, 06.12.2019 06:31



Mathematics, 06.12.2019 06:31

Mathematics, 06.12.2019 06:31