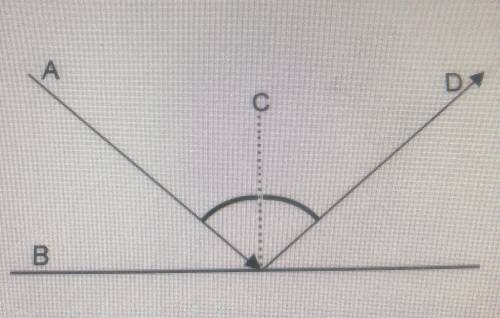
In the mirror diagram shown, Which is the normal?
...

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g 89% just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g show your calculations below. analysis questions 1. based on taste observations only, which ingredients were in excess in the lemonade samples in activity one? in activity one the excess substances for each sample were the water and sugar. 2. based on the data in activity two, which excess ingredients are affecting the taste of the lemonade in the sample batch? 3. what can just lemons, inc. do during production to reduce the amount of excess ingredients and improve the taste of their lemonade? 4. try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. 5. during factory inspection, just lemons, inc. discovered that a water valve to the lemonade mixing station was not functioning. once they repair it, more water will enter the mixing station. from what you know about the limiting and excess ingredients for current lemonade production, what advice would you give engineers about the upcoming increase in water?
Answers: 1

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1
You know the right answer?
Questions

Social Studies, 13.05.2021 23:00

Biology, 13.05.2021 23:00


Physics, 13.05.2021 23:00

Computers and Technology, 13.05.2021 23:00




Chemistry, 13.05.2021 23:00

Mathematics, 13.05.2021 23:00

Chemistry, 13.05.2021 23:00


Geography, 13.05.2021 23:00

Chemistry, 13.05.2021 23:00




Mathematics, 13.05.2021 23:00


Mathematics, 13.05.2021 23:00