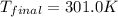Chemistry, 05.05.2020 15:04 BeautyxQueen
A 31.5 g wafer of pure gold initially at 69.4 ∘C is submerged into 63.4 g of water at 27.4 ∘C in an insulated container.
What is the final temperature of both substances at thermal equilibrium?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
A 31.5 g wafer of pure gold initially at 69.4 ∘C is submerged into 63.4 g of water at 27.4 ∘C in an...
Questions

Mathematics, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00

Physics, 30.08.2021 14:00


Mathematics, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00

English, 30.08.2021 14:00


Mathematics, 30.08.2021 14:00

Social Studies, 30.08.2021 14:00

Computers and Technology, 30.08.2021 14:00


Mathematics, 30.08.2021 14:00




Mathematics, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00

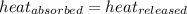
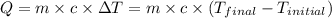
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0639/3665/09236.png) .................(1)
.................(1)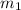 = mass of gold = 31.5 g
= mass of gold = 31.5 g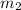 = mass of water = 63.4 g
= mass of water = 63.4 g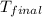 = final temperature = ?
= final temperature = ?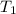 = temperature of gold =
= temperature of gold = 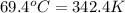
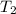 = temperature of water =
= temperature of water = 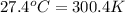
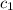 = specific heat of gold =
= specific heat of gold = 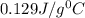
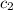 = specific heat of water=
= specific heat of water= 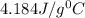
![-31.5\times 0.129\times (T_{final}-342.4)=[63.4\times 4.184\times (T_{final}-300.4)]](/tpl/images/0639/3665/eb953.png)