Chemistry, 05.05.2020 16:32 homeworkprincess
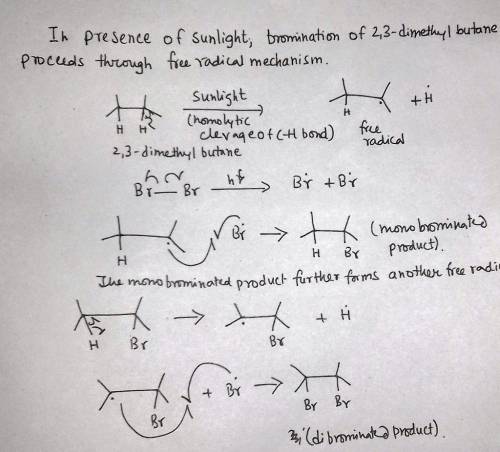
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Further reaction gives a good yield of a dibrominated product. Predict the structures of these products, and propose a mechanism for the formation of the monobrominated product.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Fu...
Questions


Mathematics, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00




Advanced Placement (AP), 13.07.2019 12:00



Mathematics, 13.07.2019 12:00


Business, 13.07.2019 12:00



English, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00


Mathematics, 13.07.2019 12:00

Biology, 13.07.2019 12:00