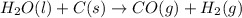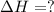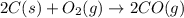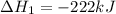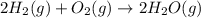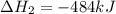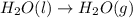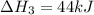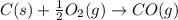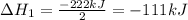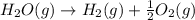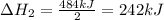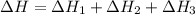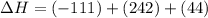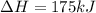Chemistry, 05.05.2020 16:29 shawn20034
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemical data above to calculate the change in enthalpy for the reaction below. H2O(l)+C(s)→CO(g)+H2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemic...
Questions

Mathematics, 01.02.2021 21:10


Chemistry, 01.02.2021 21:10


Mathematics, 01.02.2021 21:10


Health, 01.02.2021 21:10




Mathematics, 01.02.2021 21:10

Social Studies, 01.02.2021 21:10



Law, 01.02.2021 21:10




Mathematics, 01.02.2021 21:10

History, 01.02.2021 21:10