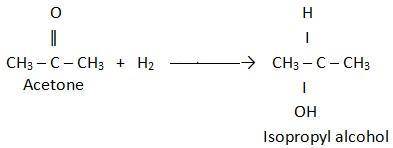
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon–oxygen double bond. calculate the enthalpy of reaction using the bond energies given. bond: c=o h–h c–h o–h c–c c–o bond energy (kj/mol): 745 432 413 467 347 358 multiple choice –61 kj +61 kj –366 kj –484 kj +366 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2


Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon–oxygen do...
Questions

Biology, 25.08.2019 13:30


Mathematics, 25.08.2019 13:30

Mathematics, 25.08.2019 13:30

Mathematics, 25.08.2019 13:30


English, 25.08.2019 13:30






Physics, 25.08.2019 13:30



Mathematics, 25.08.2019 13:30