Chemistry, 05.05.2020 16:09 alexabrandon1848
A 25.00-mL solution of 0.1500 M methylamine (CH3NH2) is titrated with a standardized 0.1025 M solution of HCl at 25°C. Enter your numbers to 2 decimal places. Kb = 4.4x10-4 What is the pH of the methylamine solution before titrant is added? 11.91 How many milliliters of titrant are required to reach the equivalence point? 36.59 What is the pH at the equivalence point?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
A 25.00-mL solution of 0.1500 M methylamine (CH3NH2) is titrated with a standardized 0.1025 M soluti...
Questions

Mathematics, 12.01.2020 14:31




English, 12.01.2020 14:31

Social Studies, 12.01.2020 14:31

Mathematics, 12.01.2020 14:31


English, 12.01.2020 14:31

English, 12.01.2020 14:31

Mathematics, 12.01.2020 14:31




Mathematics, 12.01.2020 14:31

Spanish, 12.01.2020 14:31



History, 12.01.2020 14:31

![pOH = \frac{1}{2}[pK_b \ - \ log \ C]](/tpl/images/0640/0162/873d4.png)
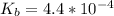
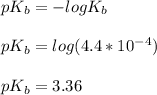
![pOH = \frac{1}{2}[3.36\ - \ log \0.15]](/tpl/images/0640/0162/71cf9.png)
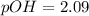
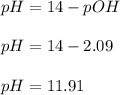
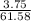
![pOH = \frac{1}{2}[pK_w+pK_b+log \ C]](/tpl/images/0640/0162/af00e.png)
![pOH = \frac{1}{2}[14+3.36+log \ 0.061]](/tpl/images/0640/0162/443df.png)
![pOH = \frac{1}{2}[16.15]](/tpl/images/0640/0162/bf604.png)