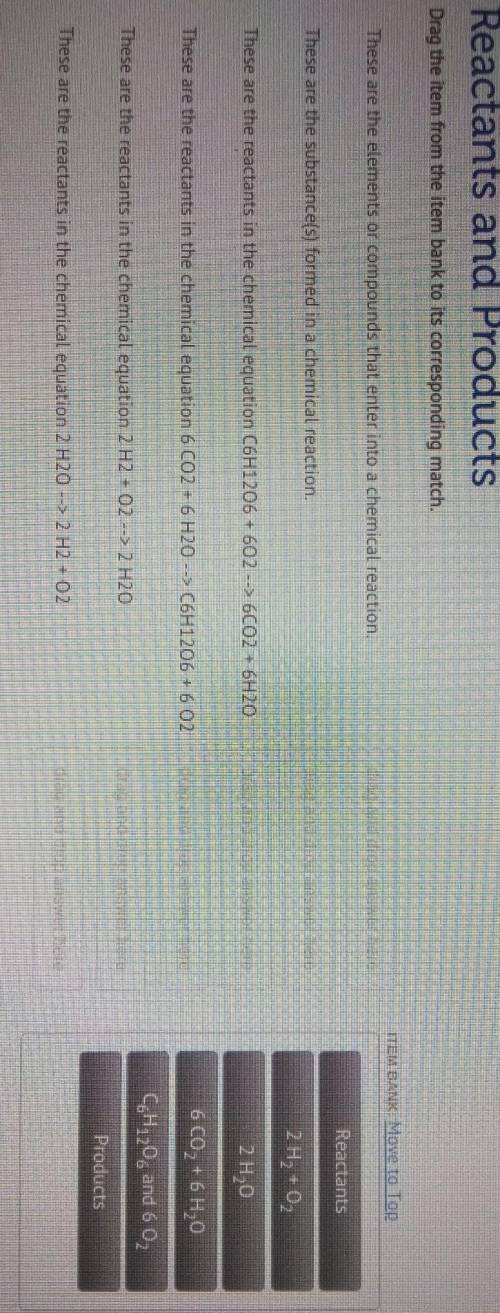
Drag the item from the item bank to its corresponding match.
ITEM BANK: Move to Top
These...

Chemistry, 05.05.2020 17:13 mjstew00763
Drag the item from the item bank to its corresponding match.
ITEM BANK: Move to Top
These are the elements or compounds that enter into a chemical reaction.
ag and drop ansaer here
Reactants
These are the substance(s) formed in a chemical reaction.
2H₂ + O₂
These are the reactants in the chemical equation C6H12O6 + 602 --> 6CO2 + 6H20
2H₂O
These are the reactants in the chemical equation 6 CO2 + 6 H20 --> C6H1206 + 6 02
6CO₂ + 6H₂O
CH120 and 6 02
These are the reactants in the chemical equation 2 H2 + 02 --> 2 H20
Products
These are the reactants in the chemical equation 2 H20 --> 2 H2 + O2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
Questions


English, 13.11.2019 21:31


Mathematics, 13.11.2019 21:31

Chemistry, 13.11.2019 21:31

Computers and Technology, 13.11.2019 21:31





Mathematics, 13.11.2019 21:31

English, 13.11.2019 21:31

Mathematics, 13.11.2019 21:31



Biology, 13.11.2019 21:31

Business, 13.11.2019 21:31

Biology, 13.11.2019 21:31

Health, 13.11.2019 21:31

History, 13.11.2019 21:31



