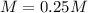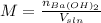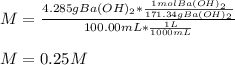Chemistry, 05.05.2020 17:05 jholland18
A solution of barium hydroxide Ba(OH) contains 4.285 g of barium hydroxide in 100.0 mL of solution. What is molarity of the solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
A solution of barium hydroxide Ba(OH) contains 4.285 g of barium hydroxide in 100.0 mL of solution....
Questions

Mathematics, 29.11.2021 02:00

Mathematics, 29.11.2021 02:00


Mathematics, 29.11.2021 02:00

Chemistry, 29.11.2021 02:00

Mathematics, 29.11.2021 02:00

Physics, 29.11.2021 02:00



Chemistry, 29.11.2021 02:00


Mathematics, 29.11.2021 02:00

History, 29.11.2021 02:00


Mathematics, 29.11.2021 02:10





History, 29.11.2021 02:10