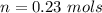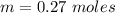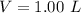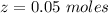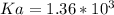Chemistry, 05.05.2020 17:06 jettskii214
A solution is prepared by dissolving 0.23 mol of nitrous acid and 0.27 mol of sodium nitrite in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the present in the buffer solution. The Ka of nitrous acid is 1.36 × 10-3.A) H2OB) H3O+C) nitrite ionD) nitrous acidE) This is a buffer solution: the pH does not change upon addition of acid or base.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
A solution is prepared by dissolving 0.23 mol of nitrous acid and 0.27 mol of sodium nitrite in wate...
Questions

Mathematics, 07.12.2021 22:20



Biology, 07.12.2021 22:20

Mathematics, 07.12.2021 22:20




Mathematics, 07.12.2021 22:30



Mathematics, 07.12.2021 22:30


Mathematics, 07.12.2021 22:30

Law, 07.12.2021 22:30


English, 07.12.2021 22:30

Chemistry, 07.12.2021 22:30

Mathematics, 07.12.2021 22:30