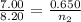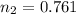Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
3.00 L of Ch4 is known to contain 0.650 moles at a certain temperature and pressure if the volume of...
Questions


English, 03.09.2020 22:01



Engineering, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

Advanced Placement (AP), 03.09.2020 22:01

Physics, 03.09.2020 22:01




English, 03.09.2020 22:01

English, 03.09.2020 22:01



Biology, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01



Mathematics, 03.09.2020 22:01

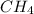 were added
were added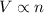
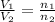
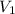 = initial volume of gas = 7.00 L
= initial volume of gas = 7.00 L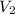 = final volume of gas = 8.20 L
= final volume of gas = 8.20 L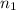 = initial moles of gas = 0.650 mole
= initial moles of gas = 0.650 mole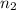 = final moles of gas = ?
= final moles of gas = ?