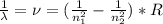Atomic hydrogen produces a well-known series of spectral lines in several regions of the electromagnetic spectrum. Each series fits the Rydberg equation with its own particular n1 value. Calculate the value of n1 that would produce a series of lines in which the highest energy line has a wavelength of 1459 nm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

You know the right answer?
Atomic hydrogen produces a well-known series of spectral lines in several regions of the electromagn...
Questions

Mathematics, 26.10.2020 19:20



English, 26.10.2020 19:20


History, 26.10.2020 19:20

Mathematics, 26.10.2020 19:20

Mathematics, 26.10.2020 19:20


Mathematics, 26.10.2020 19:20

History, 26.10.2020 19:20


Biology, 26.10.2020 19:20

Spanish, 26.10.2020 19:20



Mathematics, 26.10.2020 19:20


English, 26.10.2020 19:20

English, 26.10.2020 19:20