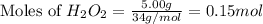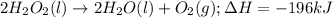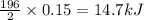Chemistry, 31.01.2020 10:05 lauren21bunch
Hydrogen peroxide can decompose to water and oxygen by the following reaction:
2h2o2 (l) 2h2o(l) + o2(g) enthalpy= -196kj
calculate the value of q when 5.00g of h20(l) decomposes at constant pressure.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Hydrogen peroxide can decompose to water and oxygen by the following reaction:
2h2o2 (l...
2h2o2 (l...
Questions


Mathematics, 23.10.2020 22:50



Mathematics, 23.10.2020 22:50

Mathematics, 23.10.2020 22:50


Social Studies, 23.10.2020 22:50


Physics, 23.10.2020 22:50

Mathematics, 23.10.2020 22:50

Mathematics, 23.10.2020 22:50

Mathematics, 23.10.2020 22:50

Mathematics, 23.10.2020 22:50

Biology, 23.10.2020 22:50



History, 23.10.2020 22:50

Mathematics, 23.10.2020 22:50

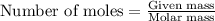
 = 5.00 g
= 5.00 g