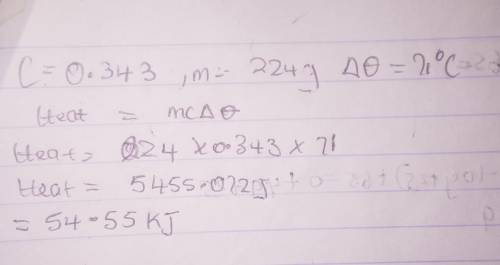Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

You know the right answer?
The specific heat capacity of an unknown metal is 0.343 J/(g•°C). Calculate the energy required to r...
Questions


English, 02.02.2020 22:00



Mathematics, 02.02.2020 22:00

Mathematics, 02.02.2020 22:00

Computers and Technology, 02.02.2020 22:00

Mathematics, 02.02.2020 22:00

Health, 02.02.2020 22:00


History, 02.02.2020 22:00



English, 02.02.2020 22:00

Mathematics, 02.02.2020 22:00


Computers and Technology, 02.02.2020 22:00

History, 02.02.2020 22:00


Mathematics, 02.02.2020 22:00