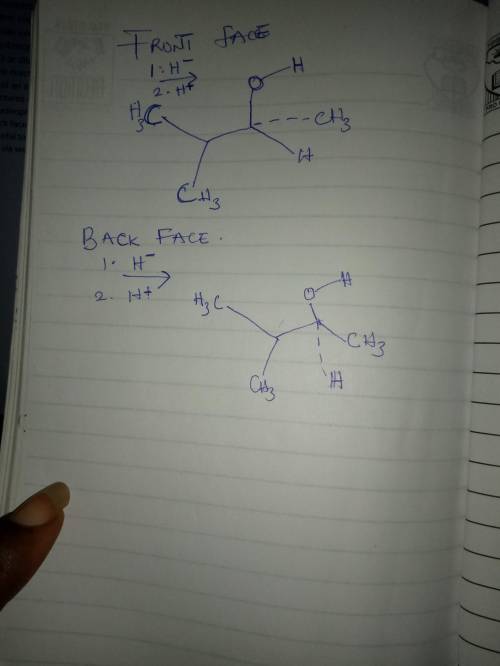
The nucleophilic addition reaction depicted below involves a prochiral ketone carbon atom reacting with a nucleophilic hydride ion source (LiAlH4 or NaBH4) and, subsequently, a proton source (e. g., H2O or dilute aq. HCl). Consequently, the reaction produces a racemic mixture of an alcohol. Finish drawing the structures of the products resulting from nucleophilic attack upon the front and back faces of the carbonyl group, being careful to specify the stereochemistry via wedge-and-dash bonds.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3


Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
The nucleophilic addition reaction depicted below involves a prochiral ketone carbon atom reacting w...
Questions

Biology, 08.07.2019 17:00

History, 08.07.2019 17:00

History, 08.07.2019 17:00

Computers and Technology, 08.07.2019 17:00

Social Studies, 08.07.2019 17:00






Mathematics, 08.07.2019 17:00


Physics, 08.07.2019 17:00

Mathematics, 08.07.2019 17:00

History, 08.07.2019 17:00

Mathematics, 08.07.2019 17:00

Biology, 08.07.2019 17:00

History, 08.07.2019 17:00


Mathematics, 08.07.2019 17:00