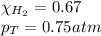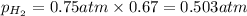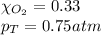Chemistry, 05.05.2020 22:21 ayoismeisjjjjuan
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hydrogen gas
(H2), and oxygen gas (O2). The mixture contains 6.7 mol hydrogen gas and 3.3 mol oxygen gas. The mixture is
in a 300 L container at 273 K and the total pressure of the gas mixture is 0.75 atm. What is the partial
pressure for each gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hy...
Questions




Physics, 20.09.2020 19:01

History, 20.09.2020 19:01




Social Studies, 20.09.2020 19:01


Mathematics, 20.09.2020 19:01






Computers and Technology, 20.09.2020 19:01



History, 20.09.2020 19:01

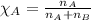
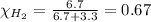
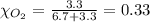
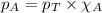
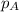 = partial pressure of substance
= partial pressure of substance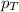 = total pressure
= total pressure = mole fraction of substance
= mole fraction of substance