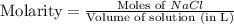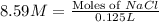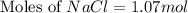How many moles of NaCl are present in a solution with a molarity of 8.59M 1 point
and 12...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
Questions


Mathematics, 28.11.2021 19:20

Mathematics, 28.11.2021 19:20

History, 28.11.2021 19:20



Mathematics, 28.11.2021 19:20

Social Studies, 28.11.2021 19:20

Mathematics, 28.11.2021 19:20

Biology, 28.11.2021 19:20

Chemistry, 28.11.2021 19:20


History, 28.11.2021 19:20



Mathematics, 28.11.2021 19:20


Mathematics, 28.11.2021 19:20

French, 28.11.2021 19:20

Computers and Technology, 28.11.2021 19:20

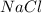 = 8.59 M
= 8.59 M