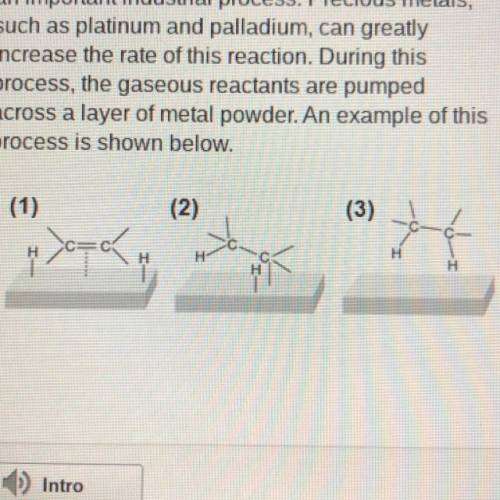
The hydrogenation of unsaturated hydrocarbons is
an important industrial process. Precious met...

Chemistry, 05.05.2020 22:59 linacelina6027
The hydrogenation of unsaturated hydrocarbons is
an important industrial process. Precious metals,
such as platinum and palladium, can greatly
increase the rate of this reaction. During this
process, the gaseous reactants are pumped
across a layer of metal powder. An example of this
process is shown below.
Describe how the metal probably increases the
reaction rate, identify whether this is an example of
homogeneous or heterogeneous catalysis, and
explain how you know.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1


Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
Questions

Mathematics, 13.04.2021 16:40

Mathematics, 13.04.2021 16:40






Mathematics, 13.04.2021 16:40


Mathematics, 13.04.2021 16:40





Mathematics, 13.04.2021 16:40



Mathematics, 13.04.2021 16:40

Mathematics, 13.04.2021 16:40

Physics, 13.04.2021 16:40



