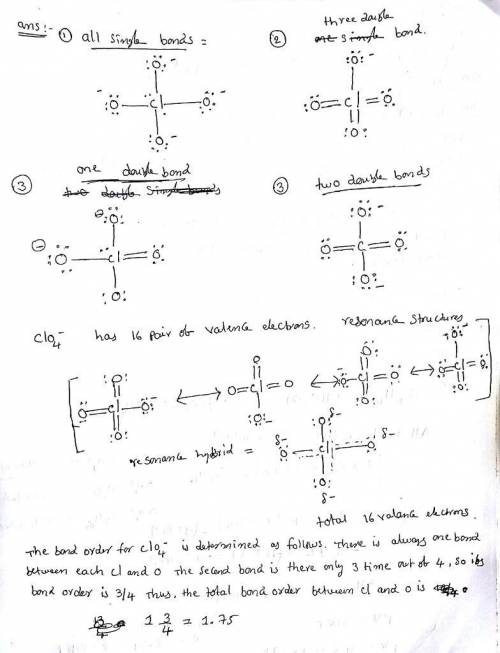
Erchlorates are powerful oxidizing agents used in fireworks, flares, and space shuttle booster rockets. Lewis structures for the perchlorate ion (ClO4−) can be drawn with all single bonds or with one, two, or three double bonds. Draw each of these possible resonance forms, including any nonbonding electrons. Include the values of any nonzero formal charges. Use formal charges to determine the most important resonance structure and calculate its average bond order.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

You know the right answer?
Erchlorates are powerful oxidizing agents used in fireworks, flares, and space shuttle booster rocke...
Questions

History, 02.12.2021 04:00




Biology, 02.12.2021 04:00


Mathematics, 02.12.2021 04:00









Computers and Technology, 02.12.2021 04:00

Chemistry, 02.12.2021 04:00

Social Studies, 02.12.2021 04:00

Mathematics, 02.12.2021 04:00

Mathematics, 02.12.2021 04:00